Date of Release: 2019
- Date of Release: 2019
- Reference link: https://www.tandfonline.com/doi/full/10.1080/09546634.2019.1692125
- Download the article file: 1564
Effect of Cedar (Ziziphus spina-christi) topical solution in mild to moderate acne vulgaris: a randomized clinical study
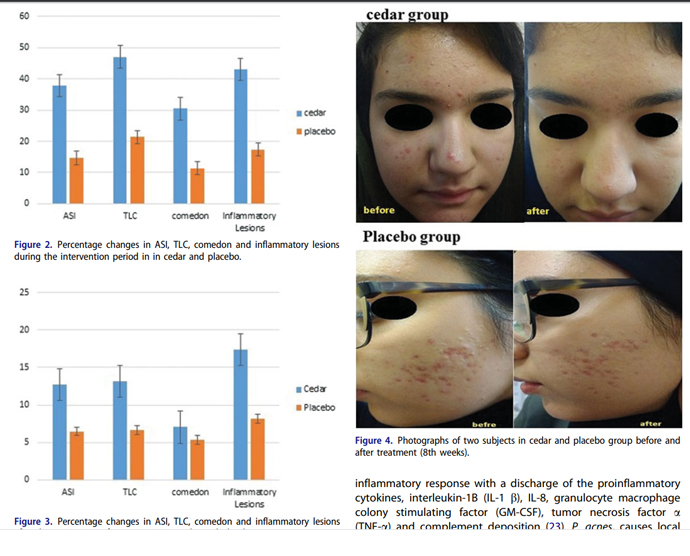
ABSTRACT Background: Acne is the most prevalent skin disease in the world and antibiotics as its standard treatments have limited and also adverse effects. Cedar (Ziziphus spina-christi) has medicinal properties like antibacterial activity and is used topically for treatment of some kinds of skin problems in Persian medicine. The aim of this study was to evaluation the efficacy of topical cedar solution of acne vulgaris Methods: Eighty patients aged between 15–45 years with mild to moderate acne vulgaris were conducted in this randomized, double blind trial. The participants were allocated to receive the topical cedar solution plus clindamycin 1% or topical placebo plus 1% clindamycin solution for six weeks Patients were evaluated at the beginning of the study, second, sixth and eighth weeks after intervention for the acne severity index (ASI) and total acne lesions counting (TLC). Data was analyzed by SPSS software with Mann–Whitney U test